NCPI FHIR Implementation Guide - Local Development build (v0.2.0). See the Directory of published versions
Resource Profile: NCPI Research Study
| Official URL: https://nih-ncpi.github.io/ncpi-fhir-ig/StructureDefinition/ncpi-research-study | Version: 0.2.0 | |||
| Draft as of 2022-10-07 | Computable Name: NcpiResearchStudy | |||
The NCPI Research Study is based upon the core FHIR ResearchStudy resource and shall act as the umbrella under which all study resources can ultimately be found. For the purposes of interoperability, some restrictions have been made to enforce a common mechanism for describing enrollment and provide a simple means for summarizing subject count and consent group membership.
Added Profile Restrictions
In order to ensure consistency across all NCPI research studies represented in FHIR, there are some additional requirements that are to be enforced:
The following requirements are true for all NCPI Research Studies:
- enrollment must contain 1 or more reference
- enrollment must only reference NCPI StudyGroup resources
- category must contain the Coding from NCPI StudyCohort
Recommended Practices
In order to support interoperability, whenever possible, the NCPI Research Study resource should have one or more Codings provided for the Study’s condition property indicating the disease or phenotype’s that were interrogated during the study’s execution.
Usage:
- Examples for this Resource Profile: ResearchStudy/ncpi-research-study-01
Formal Views of Profile Content
Description of Profiles, Differentials, Snapshots and how the different presentations work.
This structure is derived from ResearchStudy
This structure is derived from ResearchStudy
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | 0..* | ResearchStudy | Investigation to increase healthcare-related patient-independent knowledge | |
  | ||||
   | 0..* | Coding | Code defined by a terminology system Required Pattern: At least the following | |
    | 1..1 | uri | Identity of the terminology system Fixed Value: https://nih-ncpi.github.io/ncpi-fhir-ig | |
    | 1..1 | code | Symbol in syntax defined by the system Fixed Value: StudyCohort | |
   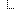 | 1..1 | string | Representation defined by the system Fixed Value: Study Cohort | |
 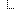 | 1..* | Reference(NCPI Study Group) | Inclusion & exclusion criteria | |
 Documentation for this format Documentation for this format | ||||
| Name | Flags | Card. | Type | Description & Constraints | ||||
|---|---|---|---|---|---|---|---|---|
 | 0..* | ResearchStudy | Investigation to increase healthcare-related patient-independent knowledge | |||||
  | Σ | 0..1 | id | Logical id of this artifact | ||||
  | Σ | 0..1 | Meta | Metadata about the resource | ||||
  | ?!Σ | 0..1 | uri | A set of rules under which this content was created | ||||
  | 0..1 | code | Language of the resource content Binding: CommonLanguages (preferred): A human language.
| |||||
  | 0..1 | Narrative | Text summary of the resource, for human interpretation | |||||
  | 0..* | Resource | Contained, inline Resources | |||||
  | 0..* | Extension | Additional content defined by implementations | |||||
  | ?! | 0..* | Extension | Extensions that cannot be ignored | ||||
  | Σ | 0..* | Identifier | Business Identifier for study | ||||
  | Σ | 0..1 | string | Name for this study | ||||
  | Σ | 0..* | Reference(PlanDefinition) | Steps followed in executing study | ||||
  | Σ | 0..* | Reference(ResearchStudy) | Part of larger study | ||||
  | ?!Σ | 1..1 | code | active | administratively-completed | approved | closed-to-accrual | closed-to-accrual-and-intervention | completed | disapproved | in-review | temporarily-closed-to-accrual | temporarily-closed-to-accrual-and-intervention | withdrawn Binding: ResearchStudyStatus (required): Codes that convey the current status of the research study. | ||||
  | Σ | 0..1 | CodeableConcept | treatment | prevention | diagnostic | supportive-care | screening | health-services-research | basic-science | device-feasibility Binding: ResearchStudyPrimaryPurposeType (extensible): Codes for the main intent of the study. | ||||
  | Σ | 0..1 | CodeableConcept | n-a | early-phase-1 | phase-1 | phase-1-phase-2 | phase-2 | phase-2-phase-3 | phase-3 | phase-4 Binding: ResearchStudyPhase (example): Codes for the stage in the progression of a therapy from initial experimental use in humans in clinical trials to post-market evaluation. | ||||
  | Σ | 0..* | CodeableConcept | Classifications for the study Binding: (unbound) (example): Codes that describe the type of research study. E.g. Study phase, Interventional/Observational, blinding type, etc. | ||||
   | 0..1 | string | Unique id for inter-element referencing | |||||
   | 0..* | Extension | Additional content defined by implementations Slice: Unordered, Open by value:url | |||||
   | Σ | 0..* | Coding | Code defined by a terminology system Required Pattern: At least the following | ||||
    | 0..1 | id | Unique id for inter-element referencing | |||||
    | 0..* | Extension | Additional content defined by implementations | |||||
    | 1..1 | uri | Identity of the terminology system Fixed Value: https://nih-ncpi.github.io/ncpi-fhir-ig | |||||
    | 0..1 | string | Version of the system - if relevant | |||||
    | 1..1 | code | Symbol in syntax defined by the system Fixed Value: StudyCohort | |||||
    | 1..1 | string | Representation defined by the system Fixed Value: Study Cohort | |||||
   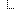 | 0..1 | boolean | If this coding was chosen directly by the user | |||||
  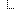 | Σ | 0..1 | string | Plain text representation of the concept | ||||
  | Σ | 0..* | CodeableConcept | Drugs, devices, etc. under study Binding: (unbound) (example): Codes for medications, devices and other interventions. | ||||
  | Σ | 0..* | CodeableConcept | Condition being studied Binding: Condition/Problem/DiagnosisCodes (example): Identification of the condition or diagnosis. | ||||
  | Σ | 0..* | ContactDetail | Contact details for the study | ||||
  | 0..* | RelatedArtifact | References and dependencies | |||||
  | Σ | 0..* | CodeableConcept | Used to search for the study Binding: (unbound) (example): Words associated with the study that may be useful in discovery. | ||||
  | Σ | 0..* | CodeableConcept | Geographic region(s) for study Binding: Jurisdiction ValueSet (extensible): Countries and regions within which this artifact is targeted for use. | ||||
  | 0..1 | markdown | What this is study doing | |||||
  | Σ | 1..* | Reference(NCPI Study Group) | Inclusion & exclusion criteria | ||||
  | Σ | 0..1 | Period | When the study began and ended | ||||
  | Σ | 0..1 | Reference(Organization) | Organization that initiates and is legally responsible for the study | ||||
  | Σ | 0..1 | Reference(Practitioner | PractitionerRole) | Researcher who oversees multiple aspects of the study | ||||
  | Σ | 0..* | Reference(Location) | Facility where study activities are conducted | ||||
  | Σ | 0..1 | CodeableConcept | accrual-goal-met | closed-due-to-toxicity | closed-due-to-lack-of-study-progress | temporarily-closed-per-study-design Binding: ResearchStudyReasonStopped (example): Codes for why the study ended prematurely. | ||||
  | 0..* | Annotation | Comments made about the study | |||||
  | 0..* | BackboneElement | Defined path through the study for a subject | |||||
   | 0..1 | string | Unique id for inter-element referencing | |||||
   | 0..* | Extension | Additional content defined by implementations | |||||
   | ?!Σ | 0..* | Extension | Extensions that cannot be ignored even if unrecognized | ||||
   | 1..1 | string | Label for study arm | |||||
   | 0..1 | CodeableConcept | Categorization of study arm | |||||
   | 0..1 | string | Short explanation of study path | |||||
  | 0..* | BackboneElement | A goal for the study | |||||
   | 0..1 | string | Unique id for inter-element referencing | |||||
   | 0..* | Extension | Additional content defined by implementations | |||||
   | ?!Σ | 0..* | Extension | Extensions that cannot be ignored even if unrecognized | ||||
   | 0..1 | string | Label for the objective | |||||
   | 0..1 | CodeableConcept | primary | secondary | exploratory Binding: ResearchStudyObjectiveType (preferred): Codes for the kind of study objective. | |||||
 Documentation for this format Documentation for this format | ||||||||
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | 0..* | ResearchStudy | Investigation to increase healthcare-related patient-independent knowledge | |
 Documentation for this format Documentation for this format | ||||
This structure is derived from ResearchStudy
Differential View
This structure is derived from ResearchStudy
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | 0..* | ResearchStudy | Investigation to increase healthcare-related patient-independent knowledge | |
  | ||||
   | 0..* | Coding | Code defined by a terminology system Required Pattern: At least the following | |
    | 1..1 | uri | Identity of the terminology system Fixed Value: https://nih-ncpi.github.io/ncpi-fhir-ig | |
    | 1..1 | code | Symbol in syntax defined by the system Fixed Value: StudyCohort | |
    | 1..1 | string | Representation defined by the system Fixed Value: Study Cohort | |
  | 1..* | Reference(NCPI Study Group) | Inclusion & exclusion criteria | |
 Documentation for this format Documentation for this format | ||||
Snapshot View
| Name | Flags | Card. | Type | Description & Constraints | ||||
|---|---|---|---|---|---|---|---|---|
 | 0..* | ResearchStudy | Investigation to increase healthcare-related patient-independent knowledge | |||||
  | Σ | 0..1 | id | Logical id of this artifact | ||||
  | Σ | 0..1 | Meta | Metadata about the resource | ||||
  | ?!Σ | 0..1 | uri | A set of rules under which this content was created | ||||
  | 0..1 | code | Language of the resource content Binding: CommonLanguages (preferred): A human language.
| |||||
  | 0..1 | Narrative | Text summary of the resource, for human interpretation | |||||
  | 0..* | Resource | Contained, inline Resources | |||||
  | 0..* | Extension | Additional content defined by implementations | |||||
  | ?! | 0..* | Extension | Extensions that cannot be ignored | ||||
  | Σ | 0..* | Identifier | Business Identifier for study | ||||
  | Σ | 0..1 | string | Name for this study | ||||
  | Σ | 0..* | Reference(PlanDefinition) | Steps followed in executing study | ||||
  | Σ | 0..* | Reference(ResearchStudy) | Part of larger study | ||||
  | ?!Σ | 1..1 | code | active | administratively-completed | approved | closed-to-accrual | closed-to-accrual-and-intervention | completed | disapproved | in-review | temporarily-closed-to-accrual | temporarily-closed-to-accrual-and-intervention | withdrawn Binding: ResearchStudyStatus (required): Codes that convey the current status of the research study. | ||||
  | Σ | 0..1 | CodeableConcept | treatment | prevention | diagnostic | supportive-care | screening | health-services-research | basic-science | device-feasibility Binding: ResearchStudyPrimaryPurposeType (extensible): Codes for the main intent of the study. | ||||
  | Σ | 0..1 | CodeableConcept | n-a | early-phase-1 | phase-1 | phase-1-phase-2 | phase-2 | phase-2-phase-3 | phase-3 | phase-4 Binding: ResearchStudyPhase (example): Codes for the stage in the progression of a therapy from initial experimental use in humans in clinical trials to post-market evaluation. | ||||
  | Σ | 0..* | CodeableConcept | Classifications for the study Binding: (unbound) (example): Codes that describe the type of research study. E.g. Study phase, Interventional/Observational, blinding type, etc. | ||||
   | 0..1 | string | Unique id for inter-element referencing | |||||
   | 0..* | Extension | Additional content defined by implementations Slice: Unordered, Open by value:url | |||||
   | Σ | 0..* | Coding | Code defined by a terminology system Required Pattern: At least the following | ||||
    | 0..1 | id | Unique id for inter-element referencing | |||||
    | 0..* | Extension | Additional content defined by implementations | |||||
    | 1..1 | uri | Identity of the terminology system Fixed Value: https://nih-ncpi.github.io/ncpi-fhir-ig | |||||
    | 0..1 | string | Version of the system - if relevant | |||||
    | 1..1 | code | Symbol in syntax defined by the system Fixed Value: StudyCohort | |||||
    | 1..1 | string | Representation defined by the system Fixed Value: Study Cohort | |||||
    | 0..1 | boolean | If this coding was chosen directly by the user | |||||
   | Σ | 0..1 | string | Plain text representation of the concept | ||||
  | Σ | 0..* | CodeableConcept | Drugs, devices, etc. under study Binding: (unbound) (example): Codes for medications, devices and other interventions. | ||||
  | Σ | 0..* | CodeableConcept | Condition being studied Binding: Condition/Problem/DiagnosisCodes (example): Identification of the condition or diagnosis. | ||||
  | Σ | 0..* | ContactDetail | Contact details for the study | ||||
  | 0..* | RelatedArtifact | References and dependencies | |||||
  | Σ | 0..* | CodeableConcept | Used to search for the study Binding: (unbound) (example): Words associated with the study that may be useful in discovery. | ||||
  | Σ | 0..* | CodeableConcept | Geographic region(s) for study Binding: Jurisdiction ValueSet (extensible): Countries and regions within which this artifact is targeted for use. | ||||
  | 0..1 | markdown | What this is study doing | |||||
  | Σ | 1..* | Reference(NCPI Study Group) | Inclusion & exclusion criteria | ||||
  | Σ | 0..1 | Period | When the study began and ended | ||||
  | Σ | 0..1 | Reference(Organization) | Organization that initiates and is legally responsible for the study | ||||
  | Σ | 0..1 | Reference(Practitioner | PractitionerRole) | Researcher who oversees multiple aspects of the study | ||||
  | Σ | 0..* | Reference(Location) | Facility where study activities are conducted | ||||
  | Σ | 0..1 | CodeableConcept | accrual-goal-met | closed-due-to-toxicity | closed-due-to-lack-of-study-progress | temporarily-closed-per-study-design Binding: ResearchStudyReasonStopped (example): Codes for why the study ended prematurely. | ||||
  | 0..* | Annotation | Comments made about the study | |||||
  | 0..* | BackboneElement | Defined path through the study for a subject | |||||
   | 0..1 | string | Unique id for inter-element referencing | |||||
   | 0..* | Extension | Additional content defined by implementations | |||||
   | ?!Σ | 0..* | Extension | Extensions that cannot be ignored even if unrecognized | ||||
   | 1..1 | string | Label for study arm | |||||
   | 0..1 | CodeableConcept | Categorization of study arm | |||||
   | 0..1 | string | Short explanation of study path | |||||
  | 0..* | BackboneElement | A goal for the study | |||||
   | 0..1 | string | Unique id for inter-element referencing | |||||
   | 0..* | Extension | Additional content defined by implementations | |||||
   | ?!Σ | 0..* | Extension | Extensions that cannot be ignored even if unrecognized | ||||
   | 0..1 | string | Label for the objective | |||||
   | 0..1 | CodeableConcept | primary | secondary | exploratory Binding: ResearchStudyObjectiveType (preferred): Codes for the kind of study objective. | |||||
 Documentation for this format Documentation for this format | ||||||||
Other representations of profile: CSV, Excel, Schematron
Terminology Bindings
| Path | Conformance | ValueSet | ||||
| ResearchStudy.language | preferred | CommonLanguages
| ||||
| ResearchStudy.status | required | ResearchStudyStatus | ||||
| ResearchStudy.primaryPurposeType | extensible | ResearchStudyPrimaryPurposeType | ||||
| ResearchStudy.phase | example | ResearchStudyPhase | ||||
| ResearchStudy.category | example | |||||
| ResearchStudy.focus | example | |||||
| ResearchStudy.condition | example | Condition/Problem/DiagnosisCodes | ||||
| ResearchStudy.keyword | example | |||||
| ResearchStudy.location | extensible | Jurisdiction ValueSet | ||||
| ResearchStudy.reasonStopped | example | ResearchStudyReasonStopped | ||||
| ResearchStudy.objective.type | preferred | ResearchStudyObjectiveType |
Constraints
| Id | Grade | Path(s) | Details | Requirements |
| ele-1 | error | **ALL** elements | All FHIR elements must have a @value or children : hasValue() or (children().count() > id.count()) | |
| ext-1 | error | **ALL** extensions | Must have either extensions or value[x], not both : extension.exists() != value.exists() |
Notes:
Each NCPI Research must have at least one NCPI Study Group which must, at the very least indicate the total number of patients enrolled at the time the data was loaded into FHIR. Additional Study Groups can be present indicating relevant groupings of subjects, such as for different consent groups.
Practices for Summary Only Resources
For Studies loaded into Summary Only FHIR servers, the Study’s Study Group resources must have the quantity. This promotes findability by enabling researches without current access to the study’s row-level data to get basic study details including the different subject counts.
For studies that exist alongside row-level data, the Study’s Study Group resources should have each corresponding Patient referenced in the Group’s members array.